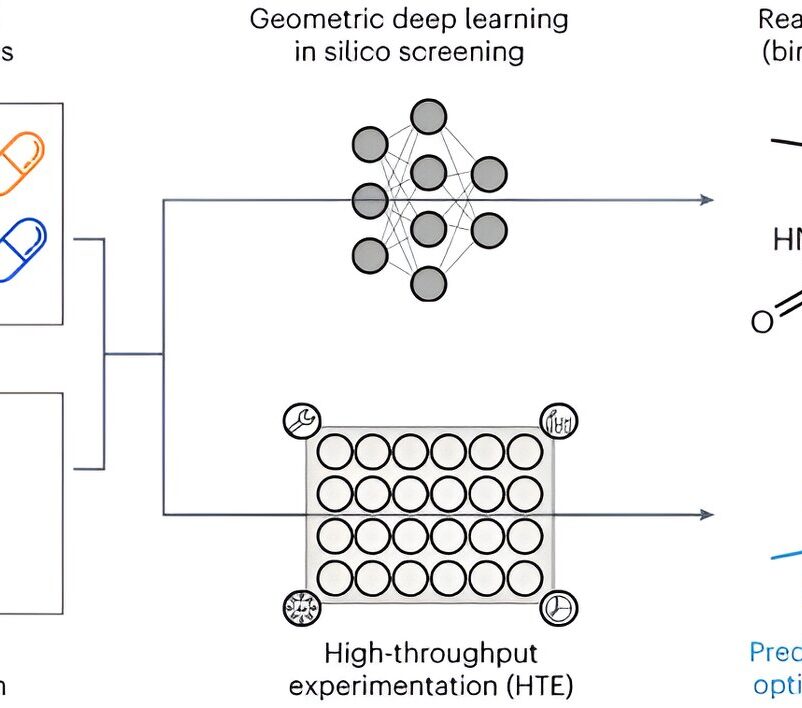
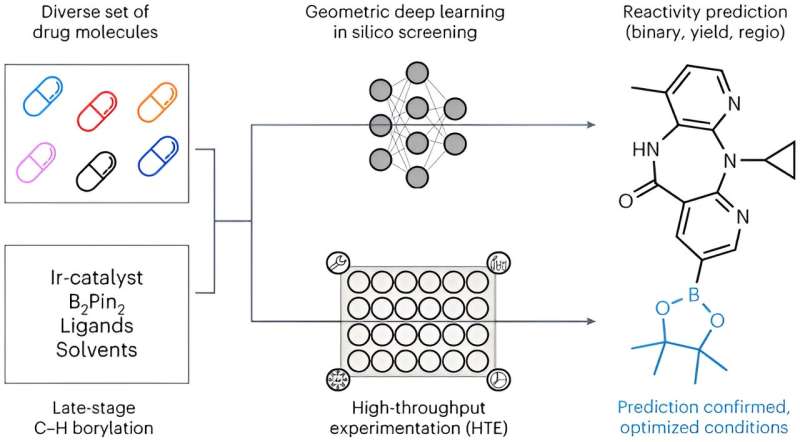
A team of researchers from LMU, ETH Zurich, and Roche Pharma Research and Early Development (pRED) Basel has used artificial intelligence (AI) to develop an innovative method that predicts the optimal method for synthesizing drug molecules.
“This method has the potential to significantly reduce the number of required lab experiments, thereby increasing both the efficiency and sustainability of chemical synthesis,” says David Nippa, lead author of the corresponding paper, which has been published in the journal Nature Chemistry. Nippa is a doctoral student in Dr. David Konrad’s research group at the Faculty of Chemistry and Pharmacy at LMU and at Roche.
Active pharmaceutical ingredients typically consist of a framework to which functional groups are attached. These groups enable a specific biological function. To achieve new or improved medical effects, functional groups are altered and added to new positions in the framework. However, this process is particularly challenging in chemistry, as the frameworks, which mainly consist of carbon and hydrogen atoms, are hardly reactive themselves.
One method of activating the framework is the so-called borylation reaction. In this process, a chemical group containing the element boron is attached to a carbon atom of the framework. This boron group can then be replaced by a variety of medically effective groups. Although borylation has great potential, it is difficult to control in the lab.
Together with Kenneth Atz, a doctoral student at ETH Zurich, David Nippa developed an AI model that was trained on data from trustworthy scientific works and experiments from an automated lab at Roche. It can successfully predict the position of borylation for any molecule and provides the optimal conditions for the chemical transformation.
“Interestingly, the predictions improved when the three-dimensional information of the starting materials were taken into account, not just their two-dimensional chemical formulas,” says Atz.
The method has already been successfully used to identify positions in existing active ingredients where additional active groups can be introduced. This helps researchers develop new and more effective variants of known drug active ingredients more quickly.
More information:
David F. Nippa et al, Enabling late-stage drug diversification by high-throughput experimentation with geometric deep learning, Nature Chemistry (2023). DOI: 10.1038/s41557-023-01360-5
Citation:
Artificial intelligence paves way for new medicines (2023, November 30)
retrieved 30 November 2023
from https://techxplore.com/news/2023-11-artificial-intelligence-paves-medicines.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.


